文献:BioMed Research International
文献链接:https://onlinelibrary.wiley.com/doi/full/10.1155/2014/129458
摘要:
The Staudinger Ligation. The reaction between a phosphine and an azide producing aza-ylide was discovered by and named after Staudinger However, the formed aza-ylide hydrolyzes in water, yielding an amine and phosphine oxide and dissociating the ligation product. In order to exploit the Staudinger reaction in aqueous, biological systems, Saxon and Bertozzi introduced an electrophilic trap in the form of a methyl ester group in the ortho position to the diphenylphosphine derivative, which ultimately captures the aza-ylide intermediate by cyclization [72]. As a result an amide bond between the phosphine derivative and the azide derivative is formed. This method is nowadays widely used for bioorthogonal ligation.
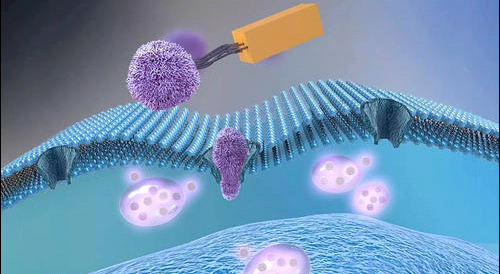
探索了无铜点击化学用于脂质体体内靶向细胞的用途。
首先,使用DSPE-PEG-NH2和磺基NHS DBCO之间的标准酰胺键形成制备DSPE-PEG-DBCO脂质(DBCO代表二苯并环辛炔)。
随后,使用由DPPC组成的脂质膜制备DBCO脂质体 : 胆固醇 : DBCO-PEG-DSPE : 用PBS水合的Cy5-DPPE(54.5:35:10:0.5摩尔比)。
A549肿瘤细胞用四酰化N-叠氮乙酰甘露糖胺(Ac4ManNAz)处理,几天后,细胞表面显示出带有附加叠氮基团的唾液酸。DBCO脂质体通过无铜点击化学成功附着在细胞表面,随后观察到肿瘤细胞对脂质体的细胞内摄取。
此外,在静脉注射DBCO脂质体前三天,通过瘤内注射Ac4ManNAz对小鼠进行治疗。这些体内研究和肿瘤组织分析的结果表明,DBCO脂质体在体内被递送到靶肿瘤。
相关推荐:
DSPE-PEG-Silane
DSPE-PEG-SP2-AA
DSPE-PEG-SS
DSPE-PEG-TCO
DSPE-PEG-Tetrazine
DSPE-PEG-TMS
DSPE-PEG-TPP
DSPE-PEG-Transferrin
DSPE-PEG-VE, MW:2000
DSPE-PEG-β-cyclodextrin
以上文章内容来源各类期刊或文献,如有侵权请联系我们删除!




 齐岳微信公众号
齐岳微信公众号 官方微信
官方微信 库存查询
库存查询