文献:Endocytic Mechanisms of Graphene Oxide Nanosheets in Osteoblasts, Hepatocytes and Macrophages
作者:Javier Linares†M. Concepción Matesanz†Mercedes Vila‡§⊥M. José Feito†Gil Gonçalves⊥María Vallet-Reg퇧Paula A. A. P. Marques⊥M. Teresa Portolés
文献链接:https://pubs.acs.org/doi/abs/10.1021/am5031598
摘要:
Nano-graphene oxide (GO) has attracted great interest in nanomedicine due to its own intrinsic properties and its possible biomedical applications such as drug delivery, tissue engineering and hyperthermia cancer therapy. However, the toxicity of GO nanosheets is not yet well-known and it is necessary to understand its entry mechanisms into mammalian cells in order to avoid cell damage and human toxicity. In the present study, the cellular uptake of pegylated GO nanosheets of ca. 100 nm labeled with fluorescein isothiocyanate (FITC-PEG-GOs) has been evaluated in the presence of eight inhibitors (colchicine, wortmannin, amiloride, cytochalasin B, cytochalasin D, genistein, phenylarsine oxide and chlorpromazine) that specifically affect different endocytosis mechanisms. Three cell types were chosen for this study: human Saos-2 osteoblasts, human HepG2 hepatocytes and murine RAW-264.7 macrophages. The results show that different mechanisms take part in FITC-PEG-GOs uptake, depending on the characteristics of each cell type. However, macropinocytosis seems to be a general internalization process in the three cell lines analyzed. Besides macropinocytosis, FITC-PEG-GOs can enter through pathways dependent on microtubules in Saos-2 osteoblasts, and through clathrin-dependent mechanisms in HepG2 hepatocytes and RAW-264.7 macrophages. HepG2 cells can also phagocytize FITC-PEG-GOs. These findings help to understand the interactions at the interface of GO nanosheets and mammalian cells and must be considered in further studies focused on their use for biomedical applications.
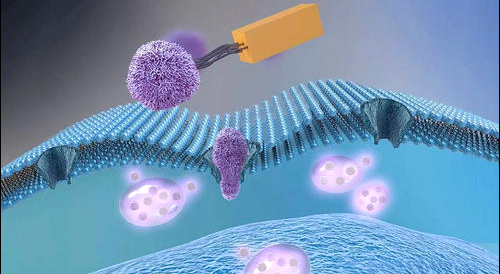
纳米氧化苯(GO)由于其固有的性质及其在药物输送、组织工程和热疗等生物医学领域的潜在应用,引起了人们对纳米医学的极大兴趣。然而,GO纳米片的毒性尚不清楚,有必要了解其进入哺乳动物细胞的机制,以避免细胞损伤和人体毒性。
在本研究中,在八种抑制剂(秋水仙素、渥曼宁、阿米洛利、细胞松弛素B、细胞松弛蛋白D、染料木素、苯胂氧化物和氯丙嗪)的存在下,评估了用异硫氰酸荧光素(FITC-PEG-GO)标记的约100nm的聚乙二醇化GO纳米片的细胞摄取,这些抑制剂专门影响不同的内吞机制。
本研究选择了三种细胞类型:人Saos-2成骨细胞、人HepG2肝细胞和小鼠RAW-264.7巨噬细胞。
结果表明,根据每种细胞类型的特征,不同的机制参与了FITC-PEG-GOs的摄取。然而,在所分析的三种细胞系中,大颗粒细胞增多似乎是一个普遍的内化过程。除了巨噬细胞作用外,FITC-PEG-GOs还可以通过Saos-2成骨细胞中依赖微管的途径进入,并通过HepG2肝细胞和RAW-264.7巨噬细胞中的网格蛋白依赖机制进入。
HepG2细胞也可以吞噬FITC-PEG-GOs。这些发现有助于理解GO纳米片和哺乳动物细胞界面上的相互作用,在进一步研究其在生物医学应用中的应用时必须加以考虑。
相关推荐:
FITC-PEG-DMG
FITC-PEG-FA
FITC-PEG-Biotin
OH-PEG-FAM
NH2-PEG-FAM
NTA-PEG-FITC
FAM-PEG-N3
FITC-PEG-AC
FITC-PEG-ACA
FITC-PEG-Alkyne
FITC-PEG-Azithromycin
FITC-PEG-CHO
FITC-PEG-DTPA
FITC-PEG-Estrogen
以上文章内容来源各类期刊或文献,如有侵权请联系我们删除!




 齐岳微信公众号
齐岳微信公众号 官方微信
官方微信 库存查询
库存查询